Principles of Boiler Water Treatment
The function of steam boiler is to evaporate water in its shell to supply steam to transfer heat energy for process. The thickness of iron to meet such demands became impracticable and mild steel for boiler construction was universally adopted in order to reduce weight by using strong material. A consequence was that corrosion became more prominent, except where the water quality was naturally “soft” and non-acidic.
Throughout the steam era, various attempts to mitigate the effect of corrosion were made, including mechanical descaling, frequent boiler wash outs, use of water softening plants and chemical dosing. The chemistry of chemical process has been understood since the turn of the 19th Century with land and marine boilers comparatively easy to control, especially where a closed circuit thermal cycle was involved.
Variations of water quality are found which, if untreated, can have differing effects on boilers. The most notable effects are;
- Corrosion of internal boiler surfaces leading to leakage and eventual failure.
- Formation of scale with a loss of thermal conductivity leading to overheating and eventual failure.
- Water carry over or priming leading to overheating and failure of the super heater and failure of the super heater, disruption of lubrication and abnormal wear of valves and control elements such as water level probe, level electrodes etc.
Water is compound of hydrogen and oxygen which has the appearance of being a clear liquid, but depending on its source, can contain impurities that vary considerably from one area of country to another. Water is often described as soft or hard depending on the concentration of calcium and magnesium salts dissolved in the water.
Where water emanates from predominantly granite sources very soft waters are found. Where the geology is predominately limestone and chalk the water is very hard. Some of waters may also be salty. Another important consideration with feed waters is the acidity and oxygen content and with mains water, its chlorine content.
The total weight of solids in solution in a given volume of feed water is referred to as Total Dissolved Solids (TDS). The concentration of TDS is measured in parts per million (ppm). In addition to calcium and magnesium salts, there are often traces of salts and metals. Non soluble contents in the boiler water are manifest in the form of suspended matter of sludge. Scale forms when the concentration of calcium and magnesium in the water exceed the solubility of calcium carbonate and magnesium carbonate in the boiler water. If slice is also present, magnesium silicate forms which acts like a cement to form a smooth glaze with the calcium carbonate or sulphate. Also dissolved in the boiler water is oxygen, carbon dioxide and in some cases, sulphur dioxide in varying proportions. A layer of scale reduces the thermal conductivity of the boiler plates and tubes and causes overheating, distortion, weakening and corrosion of the heating surfaces.
Corrosion is a term used to describe the degradation of a metal to its oxide or salt. The factors involved in this aqueous process are the concentrations of oxygen, hydrogen ions (acidity) and dissolved salts and the presence of dissimilar metals, temperature and time.
The basic aim of boiler feed water treatment is to reduce oxygen and hydrogen ions to the absolute minimum to eliminate corrosion and enable insoluble deposits to be non-adherent.
Since the start of the twentieth century chemical engineers have experimented with the addition of sodium carbonate (soda ash) and tannin thereby providing alkalinity in the boiler water (to facilitate low Hydrogen ion levels) and oxygen absorption. Such treatment followed those used in land and marine practice. This had the effect or preventing scaling and corrosion and was developed into the” TIA ” (Treatment Integral Armand) boiler water treatment system which had a dramatic effect in reducing the amount of boiler maintenance and its associated costs. Water treatment was employed in Britain in using some system. There was one drawback however, whereby higher concentrations of TDS and sludge, which were consequent of the chemical additions, tended to cause foaming and water carry-over. The TDS had therefore to be carefully controlled and the practice of “blowing down” was found necessary to ensure that TDS levels did not lead to heavy carry-over. Anti-foaming compounds were also used at high TDS levels to reduce water carry-over.
Water alkalinity is measured in pH (Power of Hydrogen). Zero pH is associated with high acid (high hydrogen ion) concentration, pH7 is referred to as neutral and pH14 with high alkali (very low hydrogen ion) concentration.
The selection of a boiler water treatment system requires careful consideration, since with all actions there are consequent reactions. Careful judgement of the chemical tools available needs to be made bearing in mind the consequences of their use. The following discourse is aimed at describing the considerations in selecting the appropriate water treatment and the controls to be adopted to ensure safe operation of boilers. It is important to note, however, that water treatment is not a cure for normal wear and tear, mismanagement of the boiler or for failings which are consequent of poor workmanship or shortcomings of the boiler design.
Water Treatment Options:
There are two methods for the treatment of feed waters used for boilers based current world Standards such as European and British Standards.
External feed water treatment:
This is the form of a fixed water treatment plant which provides reduced impurity, chemically dosed water ready for use. In the past soda/lime plants or base –exchange softeners have been used. Modern technology has provided reverse osmosis which produces water with very few impurities.
Internal boiler water treatment:
This is where chemicals, usually soda ash (sodium carbonate) or caustic soda (sodium hydroxide) and tannin (tannic asid - C76H52O46) are added directly to the boiler or boiler feed water either as powders or solutions. Tannins are organic anti-oxidant chemical compound like polyphenol.
Polyphenol compounds with molecular weights of around 500-3000 daltons and containing enough hydroxyl groups (1-2 per 100 MW) for effective cross linking of other compounds (ASTRINGENTS). The two main types are HYDROLYZABLE TANNINS and CONDENSED TANNINS. Historically, the term has applied to many compounds and plant extracts able to render skin COLLAGEN impervious to degradation. The word tannin derives from the Celtic word for OAK TREE which was used for leather processing.
If the TDS reaches a level where carry- over occurs, suitable antifoams can be employed or the boiler water can be blow down or changed. Regular boiler water sampling is recommended and subsequent variation of the chemical dosage may be necessary to ensure that the boiler water conforms to the specification stated in Standard.
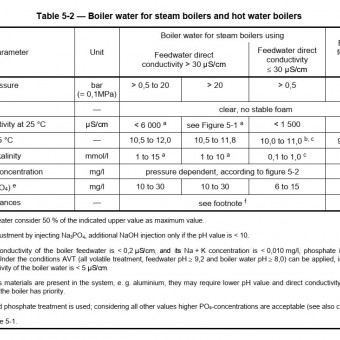
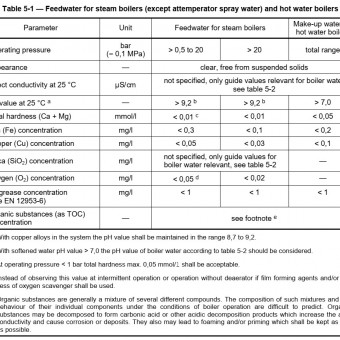
Alkalinity (pH) 10.5 – 12
Tannin (ppm) 120 – 160
Total Dissolved Solids (TDS) (ppm) 3500 max.
Departures from the above recommendations can be made, with the tannin down to 60 ppm and TDS above 3500 ppm with anti-foam, without harming the boiler.
The actual water treatment system selected depends on a number of factors. Where the treatment process is not fully understood, operators should seek professional advice from a recognised boiler water treatment specialist who has proven experience with the treatment of boilers prior to the introduction of any form of chemical addition.
The Consequences of Water Treatment:
Any operator of mechanical plant seeks to minimize cost and maximize utility, no less so than those involved with the management and operation of steam boilers. These two ideals inevitably pose a comprise.
With the use of appropriate boiler feed treatment, savings can be expected due to the following.
- Minimal corrosion of all internal boiler and steam supply line surfaces
- Elimination of scale deposists and the risk of tube and plate failures
- Improved evaporation and reduction of fuel consumption
- Purer steam production with longer lasting heater elements
- Less wear tear on the wearing surfaces of cylinders, valves and pumps
- Less boiler leakage
- Performance of burner maintained over longer periods
- Longer periods between boiler wash outs
- Longer periods between boiler maintenance and tube renewals
- Condensation tank and make up tank internal surfaces protected against corrosion
- Virtual elimination of corrosion during periods of lay up
Boiler user manual must be kept on hand at all times. All persons using chemicals should:
- Be familiar with the content of the user manual
- Know where to find them
- Be aware of how to handle the chemicals in a safe manner
- Know the appropriate treatment procedures if coming into contact wth the chemicals or treated water.
- Lead is very slowly attacked by medium alkali in the boiler water and thus fusible plugs can be copper plated to resist corrosion.
- Regular analysis needs to be carried out to assess pH, TDS and Tannin levels of the boiler water
- Dosing of the chemical additives in response to the chemical analysis needs to be carefully controlled.
- A blowdown regime is necessary to reduce excessive TDS levels is antifoams are not used.
- Wash out periods need to be optimised to remove sludge and old dislodged scale.
Boiler water with a pH above 12 must not enter the drainage system untreated and the alkalinity must be lowered before being allowed to enter the mains drainage system. Where this cannot be arranged, removal by a specialist waste disposal company may be considered.
Testing and Chemical Dosage:
It is necessary to measure the TDS, alkalinity, and tannin concentration of the boiler water. There are several methods of doing this. Possibly the easiest is to use electronic meters or specialised test kids. An accurate thermometer may also be required depending on the method in use. A source of sealable sample pots will be required together with an indelible marking medium so that samples can be retained to monitor the quality by analysis is subsequently required.
To measure pH a proprietary electronic direct read out instrument can be used. To measure TDS a simple specific gravity meter or electronic instrument can be employed. Such items are readily available from specialist suppliers. It is important to calibrate such equipment every year to ensure accuracy. Test kits are available to measure the concentration of Tannin ppm.
The amount of chemical to be added may require to be varied depending on the initial condition of the boiler. Specialist advice should be sought on this process. To calculate the dosage, the water capacity of the boiler must first be established. Following boiler wash out, the initial charge of chemicals is placed directly into the boiler and the amount will depend on the quality of the feed water being used. Subsequent additions of chemicals may be made to the water tanks following routine testing of the boiler water.
Variations in feed water quality resulting from a change in the water source or storage systems should be referred to the boiler feed treatment specialist for further analysis.
Water sample collection can be made from the water gauge drain or via a specially fitted small bore sampling valve installed, with the acceptance of the appropriate regulatory body, below the normal water level and with a small condenser coil. Sampling should be taken initially at least once during the day’s operation, however, the sampling period may be extended following experience with use of the treatment system. Results may be recorded in a log book and transferred to a permanent record sheet. The sampling procedure and record sheet should be described in the locomotive Maintenance Plan.
It is recommended that samples of boiler water should also be taken at boiler wash outs. Such samples should be retained for further analysis, if required and as a record of the boiler water contents. The clean and sealable sample pots are used for this purpose.
Points to Consider
The following supplementary points are provided for information and consideration when selecting a water treatment system.
- Water side corrosion is very limited, except under extreme conditions:
- Strong acidic
- Strong alkaline in the presence of oxygen and ammonia
- Non-aqueous fused sodium hydroxide in the presence of oxygen
- Corrosion of waterside is unlikely to occur unless use is made of contaminated water. Corrosion will occur when high concentrations of Sodium Hydroxide (caustic soda) are present in the boiler water if it leaks into rivet holes or seams. The use of suitable tannins helps to prevent this.
- A phenomenon known as “caustic embitterment” was believed to occur when steel is subject to very high alkalinity under the influence of high temperatures and stresses in excess of yield point. Caustic embitterment is a type of boiler corrosions caused by using high alkaline water in the boiler and also due to stress. This type of cracking is now thought to be caused by thermo-mechanical fatigue which is increased by excessive cold working and / or corrosive boiler water. It sometimes results in star type cracks around tube ligaments or stay holes. Failure from this mechanism can be mitigated by the use of suitable tannin compounds.
- Washouts- the principle aim of a wash out is to remove accumulated mud and scale when using water treatment the sludge formed by the chemical reactions is kept mobile and non-adherent and thus scale is not formed. The wash out period can then be safely extended, however, this depend upon the initial condition of the boiler and such extended periods between wash outs may take several seasons of experience to establish.
- Adding treatment to previously non treated boiler will gradually break up and dissolve the exinsting scale deposits. It is important to note that scale has no mechanical strength and that the treatment may well reveal wastage that was undetected in the past. Actions will be required to replace any thinned, pitted areas or badly fitting components such as tubes and connection pipes. Water treatment will not cause further leakage, in fact problems should be virtually eliminated once the remedial work has been completed and treatment has become established.
- Protection during lengthy periods out of service has traditionally involved emptying the boiler and allowing dry air to circulate. Gradual degradation is inevitable, especially in the presence of condensation. Any scale or sludge left behind may also harden out. Once a boiler is on treatment other options become available which can reduce the risk of corrosion to almost zero. These involve thoroughly washing out the boiler as a first step.
- Water can then be added with appropriate treatment to ensure sufficient alkalinity and filled to exclude all trapped air. It is also possible to add a nitrogen cap to eliminate subsequent air ingress. Such precautions must not to be employed if there is any risk of freezing as the freezing point of treated water is not much below ordinary water.
- Where risk of freezing is unavoidable, a treated boiler which its internal steel surfaces can be left under cover with dry air circulating for extended periods of time with minimal risk of corrosion.
- Blowing down, by reducing the gauge glass levels by typically a quarter or half, ,s associated with treatments where antifoaming chemicals are not used as a means of reducing the levels of TDS. Blow downs at the main blow down are also used to expel sludge accumulation. Blow down should only be carried out under controlled conditions at the place of maintenance unless a recognised continuous blow down system is employed.
- Automatically blow down system; ıt works for time relay. Boiler Operator defines a specific time for blow down time. It includes automatically valves. It automatically opens and releases the valve when set.
- Automatically TDS control; it helps to control the level of melted TDS. There will be energy save by automatic blow down operation. Energy recycling system can be installed if it is requested to get more savings. There can be 5-10% energy save at fuel consumption. For arranging of TDS amounts please contact with producer of vessel.
Conclusion:
Boiler feed water treatment has become firmly established for steam plant throughout the world. It is anticipated that the contents of this section has helped to open up the understanding of the benefits of water systems and that universal adoption of treatment will yield savings for owners, maintainers and operators. Water treatment thus offers significant benefits to the maintenance, longevity and safety of boilers.
References:
Boiler Water Treatment Principles and Practice - Colin Frayne
EN 12953 – Part 10 Requirements for feed water and boiler water quality
BS 2486 – The treatment of water for steam boilers and water heaters
Pubchem. Open chemistry database.
Itimat boiler user guide of steam boiler